Overview
The STREAM (Evaluation of a Standardized Treatment Regimen of Anti-tuberculosis Drugs for Patients with Multidrug-resistant Tuberculosis) clinical trial is the first large-scale, multi-country clinical trial to examine shortened regimens for MDR-TB. It is also the first phase III trial to test the efficacy and safety of bedaquiline in a shorter treatment regimen. In addition to evaluating the efficacy, safety and health economics of new MDR-TB regimens, STREAM has supported a robust program of community engagement, particularly at Stage 2 sites, and encouraged use of STREAM data for analyses of important secondary research questions.
The STREAM trial is comprised of two stages. Stage 1 began in 2012 as a pragmatic clinical trial. Stage 2, which added two bedaquiline-containing arms, resulted in STREAM becoming a United States Food and Drug Administration-regulated registration trial. The two stages of the trial recruited more than 1,000 participants at sites in Ethiopia, Georgia, India, Moldova, Mongolia, South Africa, Uganda, and Vietnam, making STREAM the world’s largest recruited clinical trial for MDR-TB.
“We appreciate the significant work done by the (STREAM) study investigators to generate randomized controlled clinical trial evidence for the shorter regimen. Having evidence-based policies is key to improving care for patients affected by the global public health crisis of MDR-TB.”
– Dr. Tereza Kasaeva, Director of WHO’s Global TB Programme
STREAM Stage 1
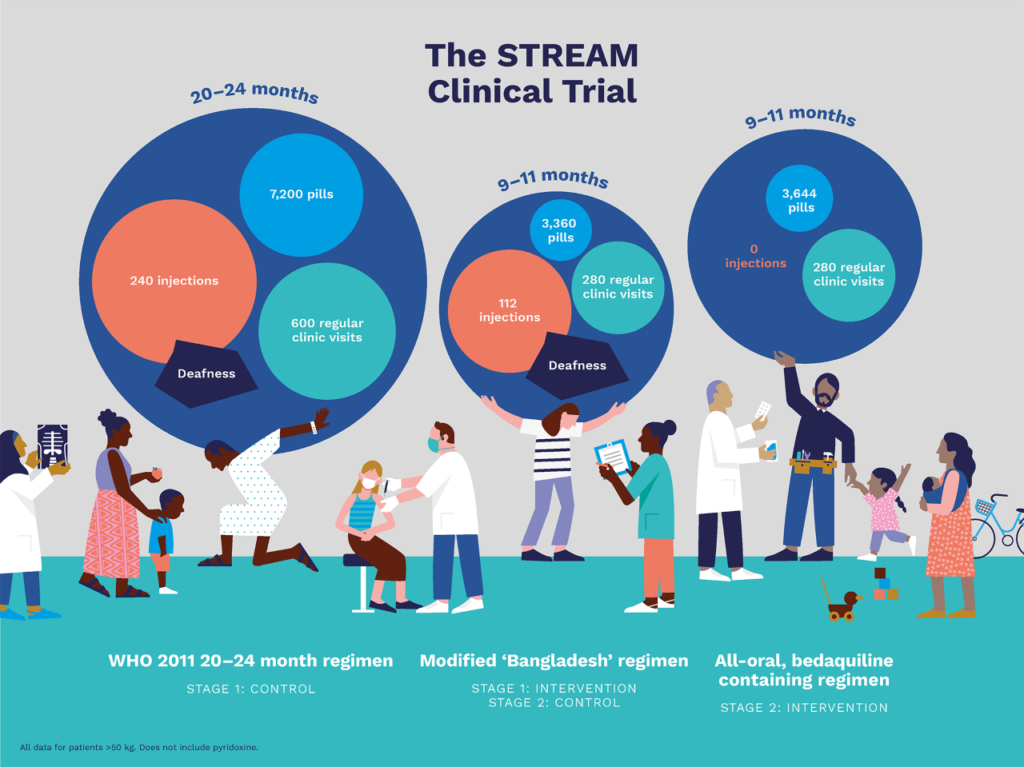
STREAM Stage 1 compared a 9–11-month MDR-TB regimen to the locally-used regimen in line with 2011 World Health Organization (WHO) guidance (approximately 20 months). The aim of Stage 1 of the STREAM trial was to evaluate the efficacy, safety and cost of the two regimens.
Stage 1 contributed important evidence to support the introduction of shorter MDR-TB regimens. The results of STREAM Stage 1, published in the New England Journal of Medicine, showed that the 9–11-month regimen was statistically non-inferior to the 20-month regimen, in terms of efficacy (78.8 % of assessable participants had a favorable outcome, compared to 79.8% in the longer regimen). Following release of results from Stage 1, the WHO acknowledged the importance of STREAM.
In early 2020, the final results of the STREAM Stage 1 health economics evaluation were published online in the Bulletin of the World Health Organization. The Stage 1 health economics evaluation demonstrated that the shorter 9–11-month regimen significantly reduced the cost of treating MDR-TB for both patients and health systems compared to the 20-month regimen.
STREAM Stage 2
STREAM Stage 2 commenced in 2016 and enrolled participants in 13 sites across Ethiopia, Georgia, India, Moldova, Mongolia, South Africa, and Uganda. Stage 2 of STREAM evaluated the efficacy, safety and cost of a 9-month all oral, bedaquiline-containing regimen and a 6-month, injectable- and bedaquiline-containing regimen, compared to the 9–11-month short regimen evaluated in Stage 1.
Bedaquiline-containing, all-oral MDR-TB regimens like the one being evaluated in Stage 2 are already being introduced based on the experience of NTPs, but there is currently no published clinical trial evidence regarding their efficacy, safety, or cost-effectiveness. Stage 2 of STREAM will help address this gap by generating high-quality evidence that could influence future guidelines and decision-making about MDR-TB treatments.
Results of the primary endpoint of Stage 2 are available here.

Achievements
The STREAM clinical trial has already influenced policy and program decisions about the use of shorter treatment regimens for MDR-TB at both the global and national level.
In early 2012, when the trial began, the standard of care for MDR-TB lasted up to 24 months, included an injectable agent, and had an average success rate of just over 50%. Although the WHO began recommending shorter treatment regimens in 2016, the Guidelines Development Group acknowledged the very low certainty in the evidence underpinning their recommendations due to a lack of randomized clinical trial data. Results from Stage 1 of STREAM filled that gap, providing high-quality evidence that contributed to the WHO’s strengthened recommendations regarding the use of shorter regimens.
In 2017, following release of STREAM Stage 1 preliminary results, the WHO stated: “The World Health Organization welcomes the release of the interim results from the STREAM Stage 1 MDR-TB randomised clinical trial and looks forward to the release of the final data and analysis. The STREAM trial – the first randomised controlled trial to test the efficacy, safety and economic impact of a standardised shorter MDR-TB regimen – demonstrates the value and importance of assessing treatment regimens in Phase III clinical trials to fully understand their potential and limitations.”
When the WHO confirmed its recommendation for use of a standardized shorter treatment regimen in a December 2018 update to the MDR-TB treatment guidelines, it again cited STREAM results as an important source underlying its recommendation.
In addition to its impact on treatment recommendations, the trial, which was implemented over a period of 10 years across eight countries, has offered an exceptional opportunity to evaluate key operational successes and lessons learned related to trial design and implementation. To read more about those lessons learned, read the “Implementing Clinical Trials” Practical Recommendations guide.
Stream Clinical Trial Resources
Practical recommendations from the STREAM clinical trial: